Calculate Concentration Of Final Solution
Concentration liquid Concentration of solution formula 5 easy ways to calculate the concentration of a solution
Concentration of solution Formula
Dilution determining final concentration (example) How to calculate concentration of acids and alkalis? Concentration calculate
Concentration solutions calculating solution mass solute percent presentation ppt powerpoint
Concentration initial dilution chemistry example calculations solvingConcentration calculations solution solutions aqueous ppt formula powerpoint presentation used following Solved: calculate the final concentration of the solution5 easy ways to calculate the concentration of a solution.
Concentrations calculating mol dm3 concentration calculate volume mass solution dm equation ppt powerpoint presentationConcentration solution calculate Solved concentration calculate solution final transcribed problem text been show hasConcentration calculate acids solution alkalis two dm units shows figure.

5 easy ways to calculate the concentration of a solution
Calculate concentrationConcentration initial stock diluted standard determine concentrations equilibrium calculate sample tube molar reaction determination chemical colorimetric analysis second scn each G formula chemistrySolutions cheat sheet.
Concentration gcseConcentration solution calculating standardized Concentration solutions sheet formula solution formulas examples cheat percent chemistry solute solvent calculations if propertiesChemistry 11: dilution calculations: solving for initial concentration.

Concentration calculate example
How to calculate concentrations when making dilutionsSolved 1. calculate the final concentration of a solution 5 easy ways to calculate the concentration of a solutionSolution concentration final calculate ml prepared problem show solved volume diluting transcribed text been has questions.
Concentration final dilution solving calculationsCalculate concentration (example) 5 easy ways to calculate the concentration of a solutionCalculate dilutions making concentrations dilution equation chemistry when using volume initial dummies values science.

Dilutions making calculate concentrations concentration when molarity solution final dummies so chemistry science
Concentration final dilution determining exampleCalculating concentrations volume concentration solution dm3 mol per powerpoint ppt grams presentation moles skip video measured slideserve How to calculate concentrations when making dilutionsConcentration calculate.
Calculating the concentration of a standardized solutionConcentration calculate solution mass Concentration volume solutions relationship v1 v2 c2 c1 solution final dilution mg after ml weight made ppt powerpoint presentationChem 11 unit 5: dilution calculations: solving for final concentration.

5 easy ways to calculate the concentration of a solution
.
.


5 Easy Ways to Calculate the Concentration of a Solution

Solutions Cheat Sheet | Online Chemistry Tutorials

5 Easy Ways to Calculate the Concentration of a Solution
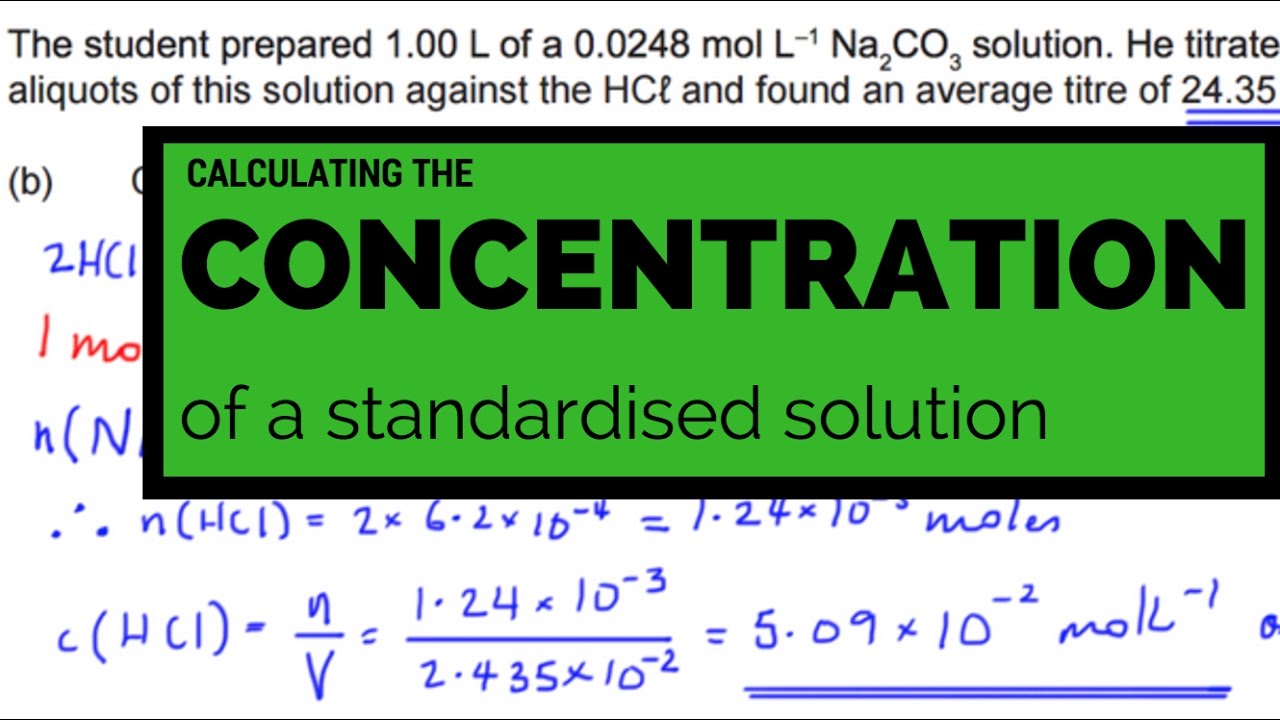
Calculating the Concentration of a Standardized Solution - YouTube

5 Easy Ways to Calculate the Concentration of a Solution

PPT - CALCULATING CONCENTRATION OF SOLUTIONS PowerPoint Presentation
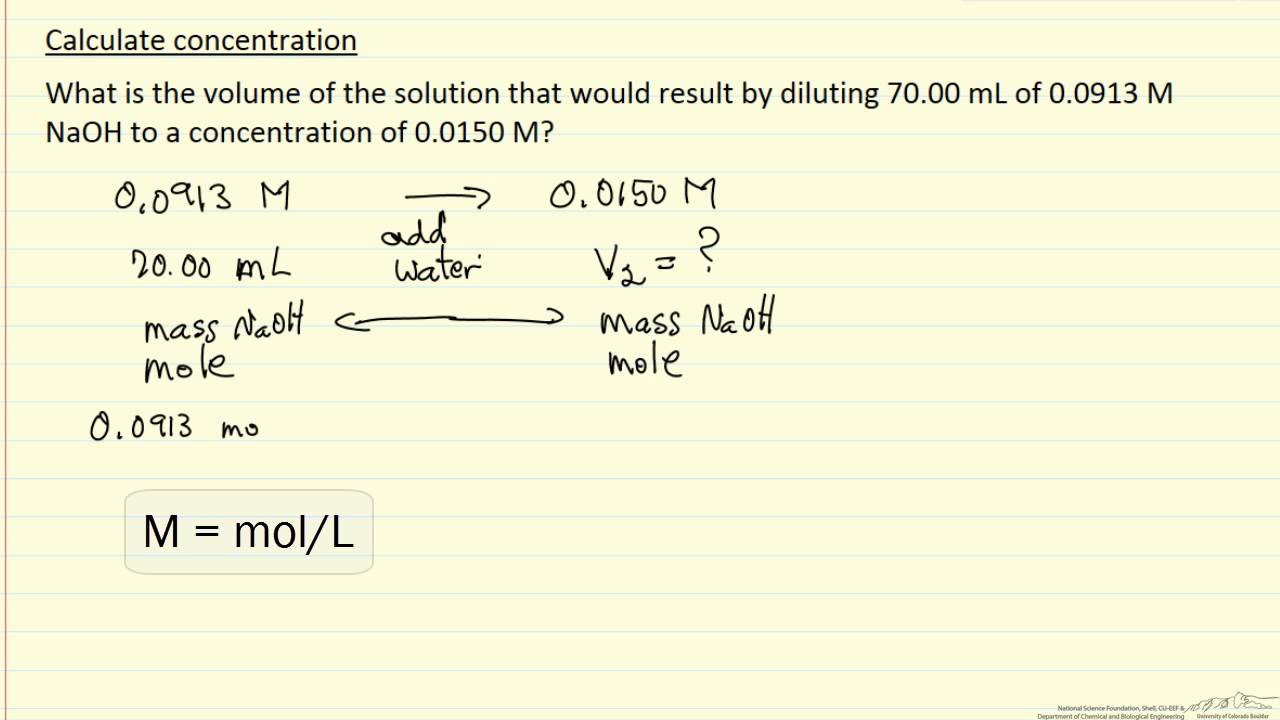
Calculate Concentration (Example) - YouTube
