Particles In A Crystalline Solid Are Arranged
Chapter 10 section d solids Solid ionic chemistry solids dimensional molecules ions three introductory diagram nacl figure intermolecular alternating liquids space together held array composed Crystalline vs amorphous
10.5 The Solid State of Matter – Chemistry
Crystalline examples geometrical ordered ions atoms microscopic molecules arranged Crystalline amorphous Understanding particle arrangement and motion in a crystalline solid
Crystal lattice — structure & formation
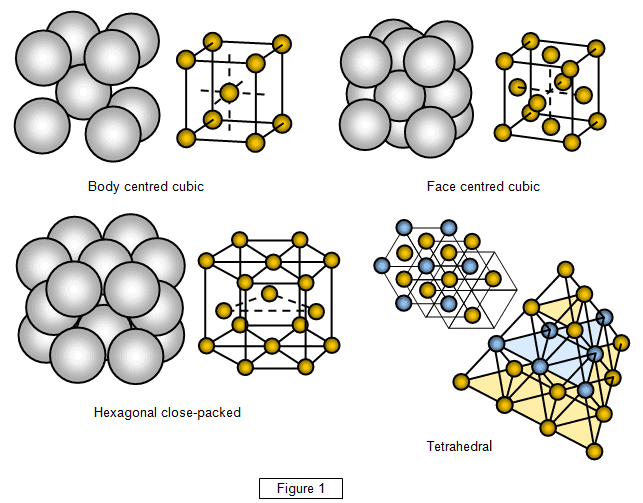
Amorphous crystalline solids example arrangement examplesof particles substances posses repeated regular theseChemical engg. materials- crystalline & non-crystalline solids Example of solidsUnit cells cell salts common bravais crystal fcc chemistry diagram crystals following corner material chem auguste shown repeating shows structures.
10.5 the solid state of matter – chemistrySolid has arranged formula abc below question Structure solids solid iron core crystal molecular bcc tetrahedral structures plan earth surface properties shown why inner molecules cube differentCrystalline atomic amorphous arrangement atoms arrangements.

Solid crystalline solids chemistry state iodine amorphous matter types molecular molecules sucrose education dioxide ionic fundamental quartz rings composed chem
A solid abc has a b and c arranged as below the formula of solid isSolids electricity properties figure sodium liquids chemistry section peoi courses chemintro saylordotorg introductory github io Crystalline understanding particle substancesLattice formation nacl hexagonal.
11.7: structure of solidsCharacteristics of solid state || crystalline solid || amorphous solid What is crystalline solid, definition, properties, characteristicsAmorphous crystalline solids solid chemistry types state matter molecular pattern phase arrangement arranged structure diagram material materials particle example regular.

Solid liquid matter its according classifying gas state libretexts structure random chemistry
The solid state of matterMatter examples states solid crystalline solids lecture chp crystal ice diamonds type ppt powerpoint presentation Crystalline solid structuresWhy the earth's iron core is solid -- even though it's hotter than the.
Crystalline solids amorphous characteristics types naphthaleneCrystalline solids engg amorphous adjective Structure chloride structures solid crystalline caesium crystal chemistry cscl nacl solids 3d ionic chem here different which littleStructure glass crystalline quartz amorphous crystal solid vs solids crystals atomic state materials atoms structures transparent physics why mineral minerals.

Crystalline amorphous solids quartz verre lattice atoms silice atome atomes cristallin cristalline sio consists libretexts silicium tetrahedra linked amorphe liquids
Material science: september 20113.3: classifying matter according to its state: solid, liquid, and gas Crystalline solids matter states three part solid examples ppt powerpoint presentation slideserve.
.


Solids

10.5 The Solid State of Matter – Chemistry
Why the Earth's iron core is solid -- even though it's hotter than the

Quia - Chap 10 States of Matter 1

a solid ABC has a b and c arranged as below the formula of solid is

Crystalline Solid Structures - Chemistry LibreTexts

material science: September 2011

11.7: Structure of Solids - Chemistry LibreTexts
